0
-
An empty cart
You have no item in your shopping cart
envato-wordpress-toolkit domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /var/www/wp-includes/functions.php on line 6121g5plus-darna domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /var/www/wp-includes/functions.php on line 6121Steam is a universal processing fluid, for the generation of electric power, as a heating medium or as a process input. Most boiler circuits involve recycle of some or all of the condensed steam after use. A typical circuit (Figure 4.18 ) involves:
1. raw or mains water intake, to make up for steam consumed or lost
2. feed water purification, including chemical dosing
3. feed water pumps, creating the steam raising pressure (which can be anything
up to and including supercritical pressures)
4. the boiler itself, in which most of the feed water is vaporized on the hot heat
transfer surfaces within the boiler
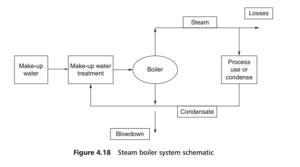
5. the process in which the steam is used, some being consumed or lost
6. a condenser for the remaining steam, converting it back to water
7. condensate recycle system, returning the condensate to the feed water purification system, and
8. a blowdown system, for draining the boiler.
Apart from the pressure (and temperature) of the generated steam, which may dictate the whole nature of the boiler plant, and certainly will dictate the required degree of purification of the feed water, the most important characteristic of the system is the composition of the make-up water, since this will determine what level of purification will be needed, and what purity of steam is likely to be generated.
The prime purpose of boiler feed water treatment is to protect the boiler, and in particular the heating surfaces, from corrosion and deposition of solids on them – the purity of the steam is largely taken care of by the evaporation of the water,
which leaves behind any residual impurities in the boiler. Therefore the treatment processes have to deal with a number of contaminants in the make-up water, and these processes may be either external ones, carried out in the treatment plant, or
internal, which conditions the water or steam in the feed lines or in the boiler itself.
The feed water treatment plant has to remove any residual suspended solids in the make-up water, and as much as possible of the dissolved solids. There should be very little suspended material, especially if the make-up water comes from a
mains supply, and it would probably be removed in the other treatment processes – so the first step is usually one of microfiltration, as much to keep the passage ways clear in subsequent treatment steps as to protect the boiler.
Dissolved material is much more of a problem, especially if the make-up water is hard. Hardness is a characteristic of groundwater that has percolated through underground strata, dissolving soluble material as it travels. Salts of calcium and magnesium (carbonates, bicarbonates, sulphates, etc.) are reasonably soluble in cold water, but progressively less so as the water temperature rises. As the water is heated in the boiler, therefore, these salts would come out of solution and deposit
as scale on the heat transfer surfaces (as in the furring of a kettle), rapidly rendering the boiler ineffective. So hard water must be softened, i.e. the dissolved materials that cause hardness must be removed. For most of the industrial history
of boilers, softening was effected by the addition of chemicals that precipitated the calcium and magnesium at ambient temperatures, so that they could be filtered out of the water. This was done in large clarification tanks with reaction chambers at the feed end (reactor clarifiers), followed by dewatering and disposal of the resultant sludge.
By far the most popular method for water softening nowadays is some form of ion removal system, such as ion exchange, electrodeionization or reverse osmosis. It must be remembered that simple ion exchange does as its name implies, that is it
exchanges potentially insoluble ions for readily soluble ones (usually sodium), so that the total ion content of the water remains the same, and deposits will still form on the tubes as the water evaporates in the boiler. The safe method of ion exchange is then an acid-base exchange system, which replaces the cations in solution with hydrogen ions, and the anions with hydroxyl groups, i.e. forming a molecule of water for every molecule of metal salt that is removed.
Complete ion removal is also offered by reverse osmosis, in principle, although the permeation of ions through even the tightest membrane is not zero, so there will be a finite, if very low, metal salt content in the purified water. If extremely low
salt contents are required, then the most cost-effective method is probably reverse osmosis followed by a deionization process.
The presence of organic materials, especially if the make-up water is from surface sources, will interfere with steam production by the formation of foams. These and other colloidal materials can be removed by ultrafiltration, which will also
remove silica, a very uncompromising deposit forming material.
The other major separation task in boiler feed water treatment is the removal of dissolved gases, especially oxygen and carbon dioxide, which will cause corrosion in the boiler. These can be removed by mechanical de-aerators, or chemicals may
be added that will scavenge these gases.
Partly because the purification processes are not completely perfect, and partly because of the build-up within the very aggressive conditions of the boiler of solids deposited from solution, it is necessary to drain accumulated solids from the boiler from time to time, in a process known as blowdown, producing a hot sludge to be dewatered and discarded.
In many steam systems, the recycled condensate makes up the largest part of the feed water. The treatment that it has already received makes it a valuable commodity, so it is not discarded, but it must be retreated, because it will pick up some
impurities in its flow back to the feed water treatment plant’ s inlet tank. Condensate polishing may be achieved by a separate ion exchange system, because of the somewhat different requirements.