0
-
An empty cart
You have no item in your shopping cart
envato-wordpress-toolkit domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /var/www/wp-includes/functions.php on line 6121g5plus-darna domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /var/www/wp-includes/functions.php on line 6121Water desalination by the reverse electrodialysis process is less commonly employed than reverse osmosis. It typically uses two types of ion exchange membrane, one allowing only the passage of negatively charged ions (anions), and the other only allows the passage of positive ions (cations). Electrodialysis was (and is) a laboratory process, used to remove salts from certain colloidal solutions, but the development of membranes with selective permeability in relation to anions and cations has totally reactivated the process.
The ions, from the feed water to be desalted, migrate towards the membranes on the application of an electric field (Figure 4.11). Anions pass through the anion-exchange membranes and cations through the cation-exchange membranes, in opposite directions. Both ion flows are then stopped by the next membrane that they reach, because it is only permeable to ions of the opposite charge. The ions thus held in alternate compartments combine to form a concentrated solution of brine.
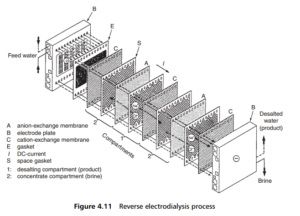
In practice an electrodialysis stack is typically composed of a large number of such compartments, separated alternately by anion- and cation-exchange membranes. The electrolyte solution in contact with the electrodes, where electrochemical reactions take place is circulated in a separate circuit. Periodically, the polarity of the electrodes is reversed, so that the ions migrate in the opposite direction, and the brine and product water compartments are interchanged. This procedure provides an automatic cleaning of the membranes, without any need for the use of cleaning chemicals.
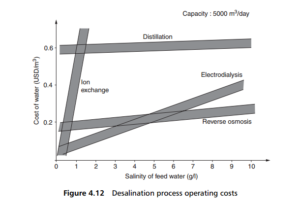
Some idea of typical operation costs of desalination processes is given in Figure 4.12, plotted against the salt concentration of the water to be desalted. Although the cost data are by now a little old, the relative costs as between the various processes are still roughly right. It can be seen that, for all but the lowest concentrations, reverse osmosis is the least expensive option.
It can be seen that the membrane has a major role to play in the provision of pure water: the removal of dissolved salts (reverse osmosis, and, more recently, nanofiltration), high quality filtration, trihalomethane compound reduction, silica
removal – all being possible with the appropriate membrane process.