0
-
An empty cart
You have no item in your shopping cart
envato-wordpress-toolkit domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /var/www/wp-includes/functions.php on line 6121g5plus-darna domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /var/www/wp-includes/functions.php on line 6121Membrane separations began in the 1960s as an alternative means to distillation for the desalination of salt (i.e. sea) and brackish waters. This was called reverse osmosis because it works by applying a transmembrane pressure greater than the natural osmotic pressure between the two solutions (seawater, say, and desalted water).
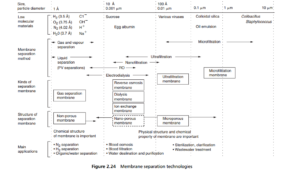
The various processes in which membranes are now used began with reverse osmosis, which, of course, is a diffusion process, in which water molecules move through the non-porous membrane, leaving the ions and other impurities behind. This is a high pressure process, needing 30–60 bar. In recent years, the ‘ tightness ’ of reverse osmosis membranes has been relaxed, to extend the diffusion separation range (see Figure 2.24 ) to allow passage of some ionic and molecular material, a process called nanofi ltration, which needs correspondingly lower transmembrane pressures (20–40 bar). True filtration is achieved at the smallest particle sizes with ultrafi ltration, which is used for the separation of large organic molecules, and colloidal solids, at pressures in the region of 5–10 bar. Membranes really entered the filtration field with the arrival of microfi ltration membranes, operating at only a few times ambient pressure. The lower pressure drops of ultrafiltration, and especially
microfiltration, enable separation with very much lower energy demands.
The microfiltration membrane is a porous one, which has the largest pore diameters among the various types of membrane. It aims at separating particles with diameters in the range of about 0.03 to 10 um (although microfiltration using other media than membranes will be used up to perhaps 100 um). This membrane separation process utilizes pressure differentials in the region of 1–5 bar, much lower than the pressures required in the diffusion-controlled processes. Microfiltration membranes are being increasingly used for the separation of very fine particles, especially in sterilization by the removal of bacteria. They are being used in a wide range of applications, and also as prefilters for ultrafiltration systems.
Ultrafiltration membranes are microporous, with a separation range from about 0.005 um to about 0.1 um (5 to 100 nm), which is roughly the size range of virus particles, so that ultrafiltration is fast becoming the last step in water purification.
The process operates at pressure differentials of 5–10 bar, still well below those of reverse osmosis and nanofiltration. Ultrafiltration is used also for the separation of large organic molecules, and its capabilities are then measured by its molecular weight cut-off (MCWO) potential, enumerated in Daltons (or kD for the largest molecules). Just as microfiltration membranes are being used as prefilters for ultrafiltration, so is the latter being employed as prefiltration for reverse osmosis.
Reverse osmosis is the process of choice, certainly where energy is cheap, for the production of drinking water from salt water. It, and nanofiltration, are both
diffusion operated processes used to separate solvent (mostly water) and some ions from a solution. They are being increasingly employed in industrial separations and clarifications.
Other liquid separation systems using membranes include dialysis and electrodialysis, where the driving force is now a concentration difference between the two sides of the membrane. The main use for dialysis is in blood processing, as a kidney replacement or booster, but both processes are also being used industrially, electro dialysis especially in desalination.
Membranes are also found in gas or vapour separations. Pervaporation is used to separate one vapour component from a liquid mixture, by diffusion of the selected vapour through the membrane, to a lower pressure on the downstream side. This is particularly useful for achieving otherwise difficult separations, such as of an azeotropic mixture. Gas separation membranes, which also operate by diffusion, are becoming a major processing tool. A significant proportion of air separation plants (into oxygen and nitrogen) now achieve the separation by membranes, as do plants for recovering hydrogen and helium from petroleum refinery off-gases.
The membrane processes that have just been briefly described can be implemented, in principle, with any material (cellulosic, synthetic polymer or inorganic) or in any format (hollow fibre, spiral wound, etc.). The system design process selects the most appropriate material and format according to the process operating parameters.
The membrane processes are characterized by quite small flow channels in and through the modules. This means that adequate prefiltration must be employed, in order to ensure as long a life as possible for the final separation stage. It is not
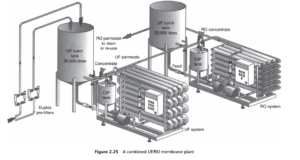
uncommon now to find, for example, an ultrafiltration plant (with its own inlet microfilters) being used as a prefilter for a reverse osmosis desalination plant, as illustrated in Figure 2.25 .
For further information, please click here.